Home » Epigenetics
Category Archives: Epigenetics
Directed Mutations, Epigenetics and Evolution
2400 words
A mutation can be said to be directed if it arises due to the needs of the developing organism, and they occur at higher frequencies if it is beneficial (Foster, 2000; Saier et al, 2017). If there is some sort of stress, then an adaptive mutation would occur. The existence of this kind of mechanism has been debated in the literature, but its existence spells trouble for neo-Darwinian theory, whose proponents claim that mutations are random and then “selected-for” in virtue of their contributions to fitness. Indeed, this concept challenges a core tenet of neo-Darwinism (Sarkar, 1991). I will argue that directed mutation/non-random mutation/stress-directed adaptation (DM, directed mutation for short) spells trouble for the neo-Darwinian paradigm.
The issue at hand
The possibility of DMs were argued for by Cairns, Overbaugh, and Miller (1988), where they argue that environmental pressure can cause adaptive changes to genes that would be beneficial to the organism. This then spurred a long debate about whether or not such mutations were possible (see Sarkar, 1991; Fox Keller, 1992; Brisson, 2003; Jablonka and Lamb, 2014). Although Cairns, Overbaugh, and Miller were wrong—that is, they were not dealing with mutations that were due to the environmental disturbances they posed (Jablonka and Lamb, 2014: 84)—their paper did bring up the possibility that some mutations could be a direct consequence of environmental disturbances which would then be catapulted by the homeodynamic physiology of the organism.
Saier et al (2017) state the specific issue with DM and its existence:
Recently, strong support for directed mutation has emerged, not for point mutations as independently proposed by Cairns, Hall and their collaborators, but for transposon-mediated mutations (12, 13). If accepted by the scientific community, this concept could advance (or revise) our perception of evolution, allowing increased rates of mutational change in times of need. But this concept goes against the current dogma that states that mutations occur randomly, and only the beneficial ones are selected for (14, 15). The concept of directed mutation, if established, would require the reversal of a long accepted precept.
This is similar to the concept of phenotypic plasticity. It is the phenomenon of a given genotype expressing different phenotypes due to environmental factors. This concept is basically a physiological one. When talking about how plastic a phenotype is, its relation to the physiology of the organism is paramount. We know that physiological changes are homeodynamic. That is, changes in physiology are constantly happening due to the effects of the environment the organism finds itself in. For example, acute changes in heart rate occur due to what happens in the environment, like say a predator chase it’s prey. The heart rates of both predator and prey increases as blood flow increases due to stress hormones. I will discuss phenotypic plasticity on its own in the future, but for now I will just note that genetic and environmental factors influence the plasticity of phenotypes (Ledon-Rettig and Ragsdale, 2021) and that phenotypic plasticity and development play a role in evolution (West-Eberhard, 2003, 2005; Wund, 2015
The fact of the matter is, phenotypic plasticity is directly related to the concept of directed mutation, due to DM being a largely physiological concept. I will argue that this refutes a central Darwinian premise. Namely that since directed mutations are possible, then they are not random. If they are not random, then due to what occurs during the development of an organism, a directed mutation could be adaptive. This, then, is the answer to how phenotypic traits become fixed in the genome without the need for natural selection.
Directed mutations
Sueoka (1988) showed that basically all organisms are subject to directed mutations. It has been noted by mathematicans that on a purely random mutational model, that there would not be enough time to explain all of the phenotypic diversity we see today (Wright, 2000). Doubt is placed on three principles of neo-Darwinism: mutations occur independently of the environment the organism is in (this is empirically false); mutations are due to replication errors (this is true, but not always the case) and mutation rates are constant (Brisson, 2003).
One of the main claims of the neo-Darwinian paradigm is that mutations occur at random, and the mutation is selected-for or against based on its relationship to fitness. Fodor’s argument has refuted the concept of natural selection, since “selection-for” is an intensional context and so can’t distinguish between correlated traits. However, we know now that since physiology is sensitive to the environment, and since adaptive changes to physiology would occur not only in an organism but during its development, it then follows that directed mutations would be a thing, and so they wouldn’t be random as neo-Darwinian dogma would claim.
In her review Stress-directed adaptive mutations and evolution, Wright (2004) concludes:
In nature, where cell division must often be negligible as a result of multiple adverse conditions, beneficial mutations for evolution can arise in specific response to stressors that target related genes for derepression. Specific transcription of these genes then results in localized DNA secondary structures containing unpaired bases vulnerable to mutation. Many environmental stressors can also affect supercoiling and [stress-directed mutation] directly.
But what are the mechanisms of DMs? “Mechanism” in this meaning would “refer to the circumstances affecting mutation rates” (Wright, 2000). She also defines what “random” means in neo-Darwinian parlance: “a mutation is random if it is unrelated to the metabolic function of the gene and if it occurs at a rate that is undirected by specific selective conditions of the environment.” Thus, the existence of DMs would then refute this tenet of neo-Darwinism. Two of the mechanisms of such DMs are transcriptional activation and supercoiling. Transcriptional activation (TA) can cause changes to single-stranded DNA (ssDNA) and also supercoiling (the addition of more coils onto DNA). TA can be caused by either derepression (which is a mechanism which occurs due to the absence of some molecule) or induction (the activation of an inactive gene which then becomes transcribed). Thus, knowing this, “genetic derepression may be the only mechanism by which particular environmental conditions of stress target specific regions of the genome for higher mutation rates (hypermutation)” (Wright, 2000). Such responses rely on a quick response, and this is due to the plastic phenotypes of the organism which then allow such DMs to occur. It then follows that stress-induced changes would allow organisms to survive in new environments, without a need for neo-Darwinian “mechanisms”—mainly natural selection. Thus, the biochemical mechanism for such mutations is transcriptional activation. Such stress-directed mutation could be seen as “quasi-Lamarckian” (Koonin and Wolf, 2009).
In nature, nutritional stress and associated genetic derepression must be rampant. If mutation rates can be altered by the many variables controlling specific, stress-induced transcription, one might reasonably argue that many mutations are to some extent directed as a result of the unique metabolism of every organism responding to the challenges of its environment. (Wright, 2000)
This is noted wonderfully by Jablonka and Lamb (2014: 92) in Evolution in Four Dimensions:
No longer can we think about mutation solely in terms of random failures in DNA maintenance and repair. We now know that stress conditions can affect the operation of the enzyme systems that are responsible for maintaining and repairing DNA, and parts of these systems sometimes seem to be coupled with regulatory elements that control how, how much, and where DNA is altered.
Jablonka and Lamb present solid evidence that mutations are semi-directed. Such mutations, as we have seen, are able to be induced by the environment in response to stress, which is due to our plastic, homeodynamic physiology. They discuss “four dimensions” of evolution which are DNA, epigenetic, behavioral and cultural. Their works (including their Epigenetic Inheritance and Evolution: The Lamarckian Dimension; see Jablonka and Lamb, 2015) provide solid evidence and arguments against the neo-Darwinian view of evolution. The fact of the matter is, there are multiple inheritance systems over and above DNA, which then contribute to nonrandom, directed mutations. The fact of the matter is, Lamarckism wasn’t wrong and Jablonka and Lamb have strongly argued for that conclusion. Epigenetics clearly influences evolution, and this therefore vindicates Lamarckism. Epigenetic variation can be inherited too (Jablonka and Lamb, 1989). Since phenotypic plasticity is relevant in how organisms adapt to their environment, then epigenetic mechanisms contribute to evolution (Ashe, Colot, and Oldroyd, 2021). Such changes that arise due to epigenetic mechanisms can indeed influence mutation (Meyer, 2015), and I would say—more directly—that certain epigenetic mechanisms play a part in how an adaptive, directed mutation would arise during the development of an organism. Stochastic epigenetic variation can indeed become adaptive (Feinberg and Irizarry, 2010).
Non-random mutations have been known to be pretty ubiquitous (Tsunoyama, Bellgard, and Gojobori, 2001). This has even been shown in the plant Arabidopis (Monroe et al, 2022), which shows that basically, mutations are not random (Domingues, 2023). A similar concept to DMs is blind stochasticity. Noble and Noble (2017, 2018; cf Noble, 2017) have shown that organisms harness stochastic processes in order to adapt to the environment—to harness function. A stochastic process is a state of a system that cannot be predicted even knowing the current state of said system.
Even all the way back in 1979, such changes were beginning to be noticed by evolutionists, such as Ho and Saunders (1979) who write that variations in the phenotype
are produced by interactions between the organism and the environment during development. We propose, therefore, that the intrinsic dynamical structure of the epigenetic system itself, in its interaction with the environment, is the source of non-random variations which direct evolutionary change, and that a proper study of evolution consists in the working out of the dynamics of the epigenetic system and its response to environmental stimuli as well as the mechanisms whereby novel developmental responses are canalized.
The organism participates in its own evolution (as considerations from niche construction show), and “evolutionary novelties” can and do arise nonrandomly (Ho, 2010). This is completely at-odds with the neo-Darwinian paradigm. Indeed, the creators of the Modern Synthesis ignored developmental and epigenetic issues when it came to formulating their theory. Fortunately, in the new millennium, we have come to understand and appreciate how development and evolution occur and how dynamic the physiological system itself truly is.
There have been critical takes on the concept of DM (Lenski and Mittler, 2003; Charlesworth, Barton, and Charlesworth, 2017; see Noble and Shapiro, 2021 for critique), like for example Futuyama (2017) who claims that DM is “groundless.” However, James Shapiro’s (1992; 2013, 2014) concept of natural genetic engineering states that cells can restructure their genomes so this “means viewing genetic change as a coordinated cell biological process, the reorganization of discrete genomic modules, resulting in the formation of new DNA structures” (Shapiro, 1993). DNA is harnessed by and for the physiological system to carry out certain tasks. Since development is self-organizing and dynamic (Smith and Thelen, 2003; Saetzler, Sonnenschein, and Soto, 2012) and since development is spurred on by physiological processes, along with the fact that physiology is sensitive to the goings-on of the environment that the developing organism finds itself in, then it follows that mutations can and would arise due to need, which would refute claims from neo-Darwinians who claim that mutations arise due to chance and not need.
Conclusion
It is clear that mutations can be (1) adaptive and (2) environmentally-induced. Such adaptive mutations, clearly, arise due to need and not chance. If they arise due to need and not chance, then they are directed and adaptive. They are directed by the plastic physiology of the organism which constructs the phenotype in a dialectical manner, using genes as its passive products, not active causes. This is because biological causation is multi-leveled, not one-way (Noble, 2012). There is also the fact of the matter that “genetic change is far from random and often not gradual” (Noble, 2013).
As can be seen in this discussion, adaptive, directed mutations are a fact of life, and so, one more domino of neo-Darwinism has fallen. Berkley claims that “The genetic variation that occurs in a population because of mutation is random“; “mutations are random“, but as we’ve seen here, this is not the case. Through the biological process of physiology and its relationship to the ebbs and flows of the environment, the organism’s phenotype that is being constructed by the self-organizing system can respond to changes in the cellular and overall environment and thusly direct changes in the phenotype and genes which would then enhance survival due to the environmental insult.
Lamarckism has been vindicated over the past 25 or so years, and it’s due to a better understanding of epigenetic processes in evolution and in the developing organism. Since what Lamarck is known for is the claim that the environment can affect the phenotype in a heritable manner, and since we now know that DNA is not the only thing inherited but epigenetically-modified DNA sequences are too, it follows that Lamarck was right. What we need to understand development and evolution is the Extended Evolutionary Synthesis, which does make novel predictions and predictions that the neo-Darwinian paradigm doesn’t (Laland et al, 2015).
Such directed changes in the genome which are caused by the physiological system due to the plastic nature of organismal construction refute a main premise of the neo-Darwinian paradigm. This is the alternative to neo-Darwinian natural selection, as Fodor noted in his attack on neo-Darwinism:
The alternative possibility to Darwin’s is that the direction of phenotypic change is very largely determined by endogenous variables. The current literature suggests that alterations in the timing of genetically controlled developmental processes is often the endogenous variable of choice; hence the ‘devo’ in ‘evo-devo’.
Darwin got quite a bit wrong, and it’s of no fault of his own. But those who claim that Darwin discovered mechanisms or articulated the random process of mutations quite obviously need to update their thoughts in the new millennium on the basis of new information informed by systems biologists and epigeneticists. The process of the construction of organisms is dynamic and self-organizing, and this is how phenotypic traits become fixed in populations of organisms. Plasticity is in fact a major driver of evolution along with the concept of genetic assimilation, which results in the canalization of the plastic trait which then eliminates the plastic response from the environment (Sommer, 2020). Phenotypic plasticity can have adaptive traits arise, but natural selection can’t be the mechanism of evolution due to Fodor’s considerations. Development can lead to evolution, not only evolution leading to development (West-Eberhard, 2003). In fact, development in many cases precedes evolution.
The Answer to Hereditarianism is Developmental Systems Theory
4150 words
Introduction
It is claimed that genes (DNA sequences) have a special, privileged role in the development of all traits. But once we understand what genes do and their role in development, then we will understand that the role ascribed to genes by gene-selectionists and hereditarians outright fails. Indeed, the whole “nature vs nurture” debate implies that genes determine traits and that it’s possible to partition the relative contributions to traits in a genetic and environmental way. This, however, is far from reality (like heritability estimates).
DST isn’t a traditional scientific theory—it is more a theoretical perspective on developmental biology, heredity, and evolution, though it does make some general predictions (Griffiths and Hochman, 2015). But aspects of it have been used to generate novel predictions in accordance with the extended evolutionary synthesis (Laland et al, 2015).
Wilson (2018: 65) notes six themes of DST:
Joint determination by multiple causes
Development is a process of multiple interacting sources.
Context sensitivity and contingency
Development depends on the current state of the organism.
Extended inheritance
An organism inherits resources from the environment in addition to genes.
Development as a process of construction
The organism helps shape its own environment, such as the way a beaver builds a dam to raise the water level to build a lodge.
Distributed control
Idea that no single source of influence has central control over an organism’s development.
Evolution as construction
The evolution of an entire developmental system, including whole ecosystems of which organisms are parts, not just the changes of a particular being or population.
Genes (DNA sequences) as resources and outcomes
Hereditarians have a reductionist view of genes and what they do. Genes, to the hereditarian, are causes of not only development but of traits and evolution, too. However the hereditarian is sorely mistaken—there is no a priori justification for treating genes as privileged causes over and above other developmental resources (Noble, 2012). I take Noble’s argument there to mean that strong causal parity is true—where causal parity means that all developmental resources are on par with each other, with no other resource having primacy over another. They all need to “dance in tune” with the “music of life” to produce the phenotype, to borrow Noble’s (2006, 2017) analogy. Hereditarian dogma also has its basis in the neo-Darwinian Modern Synthesis. The modern synthesis has gotten causality in biology wrong. Genes are, simply put, passive, not active, causes:
Genes, as DNA sequences, do not of course form selves in any ordinary sense. The DNA molecule on its own does absolutely nothing since it reacts biochemically only to triggering signals. It cannot even initiate its own transcription or replication. … It would therefore be more correct to say that genes are not active causes; they are, rather, caused to give their information by and to the system that activates them. The only kind of causation that can be attributed to them is passive, much in the way a computer program reads and uses databases. (Noble, 2011)
These ideas, of course, are also against the claim that genes are blueprints or recipes, as Plomin (2018) claims in his most recent book (Joseph, 2022). This implies that they are context-independent; we have known for years that genes are massively context-sensitive. The line of argument that hereditarians push is that genes are context-insensitive, that is they’re context-independent. But since DNA is but one of the developmental resources the physiological system uses to create the phenotype, this claim fails. Genes are not causes on their own.
Behavioral geneticist and evolutionary psychologist J. P. Rushton (1997: 64) claims that a study shows that “genes are like blueprints or recipes providing a template for propelling development forward to some targeted endpoint.” That is, Rushton is saying that there is context-independent “information” in genes, and that genes, in essence, guide development toward a targeted endpoint. Noah Carl (2019) claims that the hereditarian hypothesis “states that these differences [in cognitive ability] are partly or substantially explained by genetics.” When he says the differences are “partly or substantially explained by genetics”, he’s talking about “cognitive ability” being caused by genes. The claim that genes cause (either partly or substantially) cognitive ability—and all traits, for that matter—fails and it fails since genes don’t do what hereditarians think they do. (Nevermind the conceptual reasons.) These claims are laughable, due to what Noble, Oyama, Moore and Jablonka and Lamb have argued. It is outright false that genes are like blueprints or recipes. Rushton’s is reductionist in a sociobiology-type way, while Plomin’s is reductionist in a behavioral genetic type way.
In The Dependent Gene, David Moore (2002: 81) talks about the context-dependency of genes:
Such contextual dependence renders untenable the simplistic belief that there are coherent, long-lived entities called “genes” that dictate instructions to cellular machinery that merely constructs the body accordingly. The common belief that genes contain context-independent “information”—and so are analogous to “blueprints” or “recipes”—is simply false.
Genes are always expressed in context and cannot be divorced from said context, like hereditarians attempt using heritability analyses. Phenotypes aren’t “in the genes”, they aren’t innate. They develop through the lifespan (Blumberg, 2018).
Causal parity and hereditarianism
Hereditarianism can be said to be a form of genetic reductionism (and mind-brain identity). The main idea of reductionism is to reduce the whole to the sum of its parts and then analyze those parts. Humans (the whole) are made up of genes (the parts), so to understand human behavior, and humans as a whole, we must then understand genes, so the story goes.
Cofnas (2020) makes several claims regarding the hereditarian hypothesis and genes:
But if we find that many of the same SNPs predict intelligence in different racial groups, a risky prediction made by the hereditarian hypothesis will have passed a crucial test.
…
But if work on the genetics and neuroscience of intelligence becomes sufficiently advanced, it may soon become possible to give a convincing causal account of how specific SNPs affect brain structures that underlie intelligence (Haier, 2017). If we can give a biological account of how genes with different distributions lead to race differences, this would essentially constitute proof of hereditarianism. As of now, there is nothing that would indicate that it is particularly unlikely that race differences will turn out to have a substantial genetic component. If this possibility cannot be ruled out scientifically, we must face the ethical question of whether we ought to pursue the truth, whatever it may be.
Haier is a reductionist of not only the gene variety but the neuro variety—he attempts to reduce “intelligence” to genes and neurology (brain physiology). I have though strongly criticized the use of fMRI neuroimaging studies regarding IQ; cognitive localizations in the brain are untenable (Uttal, 2001, 2011) and this is because mind-brain identity is false.
Cofnas asks “How can we disentangle the effects of genes and environment?” and states the the behavioral geneticist has two ways—correlations between twins and adoptees and GWAS. Unfortunately for Cofnas, twin and adoption studies show no such thing (see Ho, 2013), most importantly because the EEA is false (Joseph, 2022a, b). GWAS studies are also fatally confounded (Janssens and Joyner, 2019) and PGS doesn’t show what behavioral geneticists need it to show (Richardson, 2017, 2022). The concept of “heritability” is also a bunk notion (Moore and Shenk, 2016). (Also see below for further discussion on heritability.) At the end of the day, we can’t do what the hereditarian needs to be done for their explanations to hold any water. And this is even before we look at the causal parity between genes and other developmental resources. Quite obviously, the hereditarian hypothesis is a gene-centered view, and it is of course a reductionist view. And since it is a reductionist, gene-centered view, it is then false.
Genetic, epigenetic, and environmental factors operate as a system to form the phenotype. Since this is true, therefore, both genetic and epigenetic determinism is false (also see Wagoner and Uller, 2015). It’s false because the genes one is born with, or develops with, don’t dictate or determine anything, especially not academic achievement as hereditarian gene-hunters would so gleefully claim. And one’s early experience need not dictate an expected outcome, since development is a continuous process. Although, that does not mean that environmental maladies that one experiences during childhood won’t have lasting effects into adulthood due to possibly affecting their psychology, anatomy or physiology.
The genome is responsive, that is, it is inert before it is activated by the physiological system. When we put DNA in a petri dish, it does nothing. It does nothing because DNA cannot be said to be a separate replicator from the cell (Noble, 2018). So genes don’t do anything independent of the context they’re in; they do what they do DUE TO the context they’re in. This is like Gottlieb’s (2007) probabilistic epigenesis, where the development of an organism is due to the coaction of irreducible bidirectional biological and environmental influences. David S. Moore, in The Developing Genome: An Introduction to Behavioral Epigenetics states this succinctly:
Genes—that is, DNA segments—are always influenced by their contexts, so there is never a perfect relationship between the presence of a gene and the ultimate appearance of a phenotype. Genes do not determine who we become, because nongenetic factors play critical roles in trait development; genes do what they do at least in part because of their contexts.
What he means by “critical roles in trait development” is clear if one understands Developmental Systems Theory (DST). DST was formulated by Susan Oyama (1985) in her landmark book “The Ontogeny of Information. In the book, she argues that nature and nurture are not antagonistic to each other, they are cooperative in shaping the development of organisms. Genes do not play a unique informational role in development. Thus, nature vs. nurture is a false dichotomy—it’s nature interacting with nurture, or GxE. This interactionism between nature and nurture—genes and environment—is a direct refutation of hereditarianism. What matters is context, and the context is never independent from what is going on during development. Genes aren’t the units of selection, the developmental system is, as Oyama explains in Evolution’s Eye:
If one must have a “unit” of evolution, it would be the interactive developmental system: life cycles of organisms in their niches. Evolution would then be change in the constitution and distribution of these systems (Oyama, 2000b)
Genes are important, of course, for the construction of the organism—but so are other resources. Without genes, there would be nothing for the cell to read to initiate transcription. However, without the cellular environment, we wouldn’t have DNA. Lewontin puts this wonderfully in the introduction to the 2000 edition of Ontogeny:
There are no “gene actions” outside environments, and no “environmental actions” can occur in the absence of genes. The very status of environment as a contributing cause to the nature of an organism depends on the existence of a developing organism. Without organisms there may be a physical world, but there are no environments. In like manner no organisms exist in the abstract without environments, although there may be naked DNA molecules lying in the dust. Organisms are the nexus of external circumstances and DNA molecules that make these physical circumstances into causes of development in the first place. They become causes only at their nexus, and they cannot exist as causes except in their simultaneous action. That is the essence of Oyama’s claim that information comes into existence only in the process of Ontogeny. (2000, 15-16)
Genes aren’t causes on their own, they are resources for development. And being resources for development, they have no privileged level of causation over other developmental resources, such as “methylation patterns, membrane templates, cytoplasmic gradients, centrioles, nests, parental care, habitats, and cultures” (Griffiths and Stotz, 2018). All of these things, and more of course, need to work in concert with each other.
Indeed, this is the causal parity argument—the claim that genes aren’t special developmental resources, that they are “on par” with other developmental resources (Griffiths and Gray, 1994; Griffiths and Stotz, 2018). Gene knockout studies show that the loss of a gene can be compensated by other genes—which is known as “genetic compensation.” None of the developmental resources play a more determinative role than other resources (Noble, 2012; Gamma and Liebrenz, 2019). This causal parity, then, has implications for thinking about trait ontogeny.
The causal parity of genes and other developmental factors also implies that genes cannot constitute sufficient causal routes to traits, let alone provide complete explanations of traits. Full-blown explanations will integrate various kinds of causes across different levels of organizational hierarchy, and across the divide between the internal and the external. The impossibly broad categories of nature vs. nurture that captured the imagination of our intellectual ancestors a century ago are no longer fit for the science of today. (Gamma and Liebrenz, 2019)
Oyama (2000a 40) articulates the casual parity thesis like this:
What I am arguing for here is a view of causality that gives formative weight to all operative influences, since none is alone sufficient for the phenomenon or for any of its properties, and since variation in any or many of them may or may not bring about variation in the result, depending on the configuration of the whole.
While Griffiths and Hochman (2015) formulate it like this:
The ‘parity thesis’ is the claim that if some role is alleged to be unique to nucleic acids and to justify relegating nongenetic factors to a secondary role in explaining development, it will turn out on closer examination that this role is not unique to nucleic acids, but can be played by other factors.
Genes are necessary pre-conditions for trait development, just as the other developmental resources are necessary pre-conditions for trait development. No humans without genes—this means that genes are necessary pre-conditions. If genes then humans—this implies that genes are sufficient for human life, but they are but one part of what makes humans human, when all of the interactants are present, then the phenotype can be constructed. So all of the developmental resources interacting are sufficient.
The nature vs. nurture dichotomy can be construed in such a way that they are competing explanations. However, we now know that the dichotomy is a false one and that the third way—interactionism—is how we should understand development. Despite hereditarian protestations, DST/interactionism refutes their claims. The “information” in the genes, then, cannot explain how organisms are made, since information is constructed dialectically between the resources and the system. There are a multiplicity of causal factors that are involved in this process, and genes can’t be privileged in this process. Thus the phrase “genetic causation” isn’t a coherent concept. Moreover, DNA sequences aren’t even coherent outside of cellular context (Noble, 2008).
Griffiths and Stotz (2018) put the parity argument like this:
In The Ontogeny of Information Oyama pioneered the parity argument, or the ‘parity thesis’, concerning genetic and environmental causes in development (see also Griffiths and Gray 1994; Griffiths and Gray 2005; Griffiths and Knight 1998; Stotz 2006; Stotz and Allen 2012). Oyama relentlessly tracked down failures of parity of reasoning in earlier theorists. The same feature is accorded great significance when a gene exhibits it, only to be ignored when a non-genetic factor exhibits it. When a feature thought to explain the unique importance of genetic causes in development is found to be more widely distributed across developmental causes, it is discarded and another feature is substituted. Griffiths and Gray (1994) argued in this spirit against the idea that genes are the sole or even the main source of information in development. Other ideas associated with ‘parity’ are that the study of development does not turn on a single distinction between two classes of developmental resources, and that the distinctions useful for understanding development do not all map neatly onto the distinction between genetic and non-genetic.
Shea (2011) tries to argue that genes do have a special role, and that is to transport information. Genes are, of course, inherited, but so is every other part of the system (resources). Claiming that there is information “in the genes” is tantamount to saying that there is a special role for DNA in development. But, as I hope will be clear, this claim fails due to the nature of DNA and its role in development.
This line of argument leads to one clear conclusion—genes are followers, they are not leaders; most evolution begins with environmentally-mediated phenotypic change, and then genetic changes occur (West-Eberhard, 2003). Ho and Saunders (1979) state that variation in organisms is constructed during development due to an interaction between genetic and non-genetic factors. That is, they follow what is needed to do by the developmental system, they aren’t leading development, they are but one party in the whole symphony of development. Development can be said to be irreducible, so we cannot reduce development to genes or anything else, as all interactants need to be present for development to be carried out. Since genes are activated by other factors, it is incoherent to talk of “genetic causes.” Genes affect the phenotype only when they are expressed, and other resources, too, affect the phenotype this is, ultimately, an argument genes against as blueprints, codes, recipes, or any other kind of flowery language one can used to impute what amounts to intention to inert DNA.
Even though epigenetics invalidates all genetic reductionism (Lerner and Overton, 2017), genetic reductionist ideas still persist. They give three reasons why genetic reductionist ideas still persist despite the conceptual, methodological, and empirical refutations. (1) Use of terms like “mechanism”, “trait”, and “interaction”; (2) constantly shifting to other genes once their purported “genes for” traits didn’t workout; and (3) they “buried opponents under repetitive results” (Panofsky, quoted in Lerner and Overton, 2017). The fact of the matter is, there are so many lines of evidence and argument that refute hereditarian claims that it is clear the only reason why one would still be a hereditarian in this day and age is that they’re ignorant—that is racist.
Genes, that is, are servants, not masters, of the development of form and individual differences. Genes do serve as templates for proteins: but not under their own direction. And, as entirely passive strings of chemicals, it is logically impossible for them to initiate and steer development in any sense. (Richardson, 2016)
DST and hereditarian behavioral genetics
I would say that DST challenges three claims from hereditarian behavioral genetics (HBG hereafter):
(1) The claim that we can neatly apportion genes and environment into different causes for the ontogeny of traits;
(2) Genes are the only thing that are inherited and that genes are the unit of selection and a unique—that is, special and privileged cause over and above other resources;
(3) That genes vs environment, blank skate vs human nature, are a valid dichotomy.
(1) HBG needs to rely on the attempting to portion out causes of traits into gene and environmental causes. The heritability statistic presumes additivity, thy is, it assumes no interaction. This is patently false. Charney (2016) gives the example of schizophrenia—it is claimed that 50 percent of the heritability of schizophrenia is accounted for by 8000 genes, which means that each SNP accounts for 1/8000 of the half of the heritability. This claim is clearly false, as genetics aren’t additive, and the additivity assumption precludes the interaction of genes with genes, and environment, which create new interactive environments. Biological systems are not additive, they’re interactive. Heritability estimates, therefore, are attempts at dichotomizing what is not dichitomizable (Rose, 2005).
An approach that partitions variance into independent main effects will never resolve the debate because, by definition, it has no choice but to perpetuate it. (Goldhaber, 2012)
This approach, of course, is the approach that attempts to partition variance into G and E components. The assumption is that G and E are additive. But as DST theorists have argued for almost 40 years, they are not additive, they are interactive and so not additive, therefore heritability estimates fail on conceptual grounds (as well as many others). Heritability estimates have been—and continue to today—been at the heart of the continuance of the nature vs nurture distinction, the battle, if you will. But if we accept Oyama’s causal parity argument—and due to the reality of how genes work in the system, I see no reason why we shouldn’t—then we should reject hereditarianism. Hereditarians have no choice but to continue the false dichotomy of nature vs nurture. Their “field” depends on it. But despite the fact that the main tool for the behavioral geneticist lies on false pretenses (twin and adoption studies), they still try to show that heritability estimates are valid in explaining trait variation (Segalowitz, 1999; Taylor, 2006, 2010).
(2) More than genes are inherited. Jablonka and Lamb (2005) argue that there are four dimensions—interactants—to evolution: genetic, epigenetic, behavioral, and symbolic. They show the context-dependency of the genome, meaning that genotype does not determine phenotype. What does determine the phenotype, as can be seen from the discussion here, is the interacting of developmental resources in development. Clearly, there are many other inheritance systems other than genes. There is also the fact that the gene as popularly conceived does not exist—so it should be the end of the gene as we know it.
(3) Lastly, DST throws out the false dichotomy of genes and environment, nature and nurture. DST—in all of its forms—rejects the outright false dichotomy of nature vs nurture. They are not in a battle with each other, attempting to decide who is to be the determining factor in trait ontogeny. They interact, and this interaction is irreducible. So we can’t reduce development to genes or environment (Moore, 2016) Development isn’t predetermined, it’s probabilistic. The stability of phenotypic form isn’t found in the genes (Moore and Lickliter, 2023)
Conclusion
Genes are outcomes, not causes, of evolution and they are not causes of trait ontogeny on their own. The reality is that strong causal parity is true, so genes cannot be regarded as a special developmental resource from other resources—that is, genes are not privileged resources. Since they are not privileged resources, we need to, then, dispense with any and all concepts of development that champion genes as being the leader of the developmental process. The system is, not genes, with genes being but one of many of the interactants that shape phenotypic development.
By relying on the false narrative that genes are causes and that they cause not only our traits but our psychological traits and what we deem “good” and “bad”, we would then be trading social justice for hereditarianism (genetic reductionism).
These recommended uses of bad science reinforce fears of institutionalized racism in America and further the societal marginalization of minority groups; these implications of their recommendations are never publicly considered by those who promulgate these flawed extensions of counterfactual genetic reductionism. (Lerner, 2021)
Such [disastrous societal] applications can only rob people of life chances and destroy social justice. Because developmental science has the knowledge base to change the life course trajectories of people who are often the targets of genetic reductionist ideas, all that remains to eradicate genetic reductionism from scientific discussion is to have sufficient numbers of developmental scientists willing to proclaim loudly and convincingly that the naked truth is that the “emperor” (of genetic reductionism) has no clothes. (Lerner, 2021: 338)
Clearly, hereditarians need the nature vs nurture debate to continue so they can push their misunderstandings about genes ans psychology. However, given our richer understanding of genes and how they work, we now know that hereditarianism is untenable, and DST conceptions of the gene and development as a whole have led us to that conclusion. Lerner (2017) stated that as soon as the failure of one version of genetic reductionism is observed, another one pious up—making it like a game of whack-a-mole.
The cure to hereditarian genetic reductionism is a relational developmental systems (RDS) model. This model has its origins with Uri Bronfenbrenner’s ecological systems theory (Bronfenbrenner and Ceci, 1994; Ceci, 1996; Patel, 2011; Rosa and Tudge, 2013. Development is about the interacting and relation between the individual and environment, and this is where RDS theory comes in. Biology, physiology, culture, and history are studied to explain human development (Lerner, 2021). Hereditarian ideas cannot give us anything like what models derived from developmental systems ideas can. An organism-environment view can lead to a more fruitful, and the organism and environment are inseparable (Jarvilehto, 1998; Griffiths and Gray, 2002). And it is for these reasons, including many, many more, that hereditarian genetic reductionist ideas should become mere sand in the wind.
Having said all that, here’s the argument:
P1: If hereditarianism is true, then strong causal parity is false.
P2: Strong causal parity is true.
C: Therefore hereditarianism must be false.
The Modern Synthesis vs the Extended Evolutionary Synthesis
2050 words
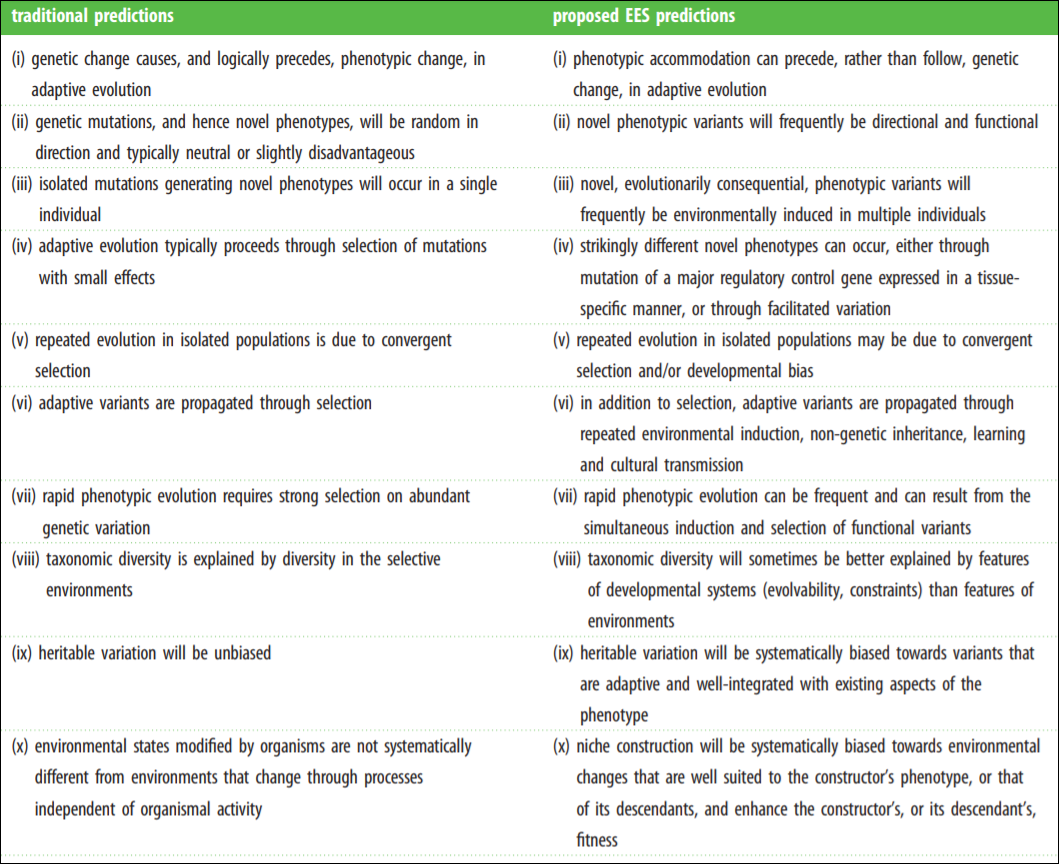
The Modern Synthesis (MS) has entrenched evolutionary thought since its inception in the mid-1950s. The MS is the integreation of Darwinian natural selection and Mendelian genetics. Key assumptions include “(i) evolutionarily significant phenotypic variation arises from genetic mutations that occur at a low rate independently of the strength and direction of natural selection; (ii) most favourable mutations have small phenotypic effects, which results in gradual phenotypic change; (iii) inheritance is genetic; (iv) natural selection is the sole explanation for adaptation; and (v) macro-evolution is the result of accumulation of differences that arise through micro-evolutionary processes” (Laland et al, 2015).
Laland et al (2015) even have a helpful table on core assumptions of both the MS and Extended Evolutionary Synthesis (EES). The MS assumptions are on the left while the EES assumptions are on the right.

Darwinian cheerleaders, such as Jerry Coyne and Richard Dawkins, would claim that neo-Darwinisim can—and already does—account for the assumptions of the EES. However, it is clear that that claim is false. At its core, the MS is a gene-centered perspective whereas the EES is an organism-centered perspective.
To the followers of the MS, evolution occurs through random mutations and change in allele frequencies which then get selected for by natural selection since they lead to an increase in fitness in that organism, and so, that trait that the genes ’cause’ then carry on to the next generation due to its contribution to fitness in that organism. Drift, mutation and gene flow also account for changes in genetic frequencies, but selection is the strongest of these modes of evolution to the Darwinian. The debate about the MS and the EES comes down to gene-selectionism vs developmental systems theory.
On the other hand, the EES is an organism-centered perspective. Adherents to the EES state that the organism is inseparable from its environment. Jarvilehto (1998) describes this well:
The theory of the organism-environment system (Jairvilehto, 1994, 1995) starts with the proposition that in any functional sense organism and environment are inseparable and form only one unitary system. The organism cannot exist without the environment and the environment has descriptive properties only if it is connected to the organism.
At its core, the EES makes evolution about the organism—its developmental system—and relegates genes, not as active causes of traits and behaviors, but as passive causes, being used by and for the system as needed (Noble, 2011; Richardson, 2017).
One can see that the core assumptions of the MS are very much like what Dawkins describes in his book The Selfish Gene (Dawkins, 1976). In the book, Dawkins claimed that we are what amounts to “gene machines”—that is, just vehicles for the riders, the genes. So, for example, since we are just gene machines, and if genes are literally selfish “things”, then all of our actions and behaviors can be reduced to the fact that our genes “want” to survive. But the “selfish gene” theory “is not even capable of direct empirical falsification” (Noble, 2011) because Richard Dawkins emphatically stated in The Extended Phenotype (Dawkins, 1982: 1) that “I doubt that there is any experiment that could prove my claim” (quoted in Noble, 2011).
Noble (2011) goes on to discuss Dawkins’ view that on genes:
Now they swarm in huge colonies, safe inside gigantic lumbering robots, sealed off from the outside world, communicating with it by tortuous indirect routes, manipulating it by remote control. They are in you and me; they created us, body and mind; and their preservation is the ultimate rationale for our existence. (1976, 20)
Noble then switches the analogy: Noble likens genes, not as having a “selfish” attribute, but to that of being “prisoners”, stuck in the body with no way of escape. Noble then says that, since there is no experiment to distinguish between the two views (which Dawkins admitted). Noble then concludes that, instead of being “selfish”, the physiological sciences look at genes as “cooperative”, since they need to “cooperate” with the environment, other genes, gene networks etc which comprise the whole organism.
In his 2018 book Agents and Goals in Evolution Samir Okasha distinguishes between type I and type II agential thinking. “In type 1 [agential thinking], the agent with the goal is an evolved entity, typically an individual organism; in type 2, the agent is ‘mother nature’, a personification of natural selection” (Okasha, 2018: 23). An example of type I agential thinking is Dawkins’ selfish genes, while type II is the personification that one imputes onto natural selection—which Okasha states that this type of thinking “Darwin was himself first to employ” (Okasha, 2018: 36) it.
Okasha states that each gene’s ultimate goal is to outcompete other genes—for that gene in question to increase its frequency in the organism. They also can have intermediate goals which is to maximize fitness. Okasha gives three rationales on what makes something “an agent”: (1) goal-directedness; (2) behavioral flexibility; and (3) adaptedness. So the “selfish” element “constitutes the strongest argument for agential thinking” of the genes (Okasha, 2018: 73). However, as Denis Noble has tirelessly pointed out, genes (DNA sequences) are inert molecules (and are one part of the developing system) and so do not show behavioral flexibility or goal-directedness. Genes can (along with other parts of the system working in concert with them) exert adaptive effects on the phenotype, though when genes (and traits) are coextensive, selection cannot distinguish between the fitness-enhancing trait and the free-riding trait so it only makes logical sense to claim that organisms are selected, not any individual traits (Fodor and Piatteli-Palmarini, 2010a, 2010b).
It is because of this, that the Neo-Darwinian gene-centric paradigm has failed, and is the reason why we need a new evolutionary synthesis. Some only wish to tweak the MS a bit in order to allow what the MS does not incorporate in it, but others want to overhaul the entire thing and extend it.
Here is the main reason why the MS fails: there is absolutely no reason to privilege any level of the system above any other! Causation is multi-level and constantly interacting. There is no a priori justification for privileging any developmental variable over any other (Noble, 2012, 2017). Both downward and upward causation exists in biological systems (which means that molecules depend on organismal context). The organism also able to control stochasticity—which is “used to … generate novelty” (Noble and Noble, 2018). Lastly, there is the creation of novelty at new levels of selection, like with how the organism is an active participant in the construction of the environment.
Now, what does the EES bring that is different from the MS? A whole bunch. Most importantly, it makes a slew of novel predictions. Laland et al (2016) write:
For example, the EES predicts that stress-induced phenotypic variation can initiate adaptive divergence in morphology, physiology and behaviour because of the ability of developmental mechanisms to accommodate new environments (consistent with predictions 1–3 and 7 in table 3). This is supported by research on colonizing populations of house finches [68], water fleas [132] and sticklebacks [55,133] and, from a more macro-evolutionary perspective, by studies of the vertebrate limb [57]. The predictions in table 3 are a small subset of those that characterize the EES, but suffice to illustrate its novelty, can be tested empirically, and should encourage deriving and testing further predictions.
[Table 3]
There are other ways to verify EES predictions, and they’re simple and can be done in the lab. In his book Above the Gene, Beyond Biology: Toward a Philosophy of Epigenetics, philosopher of biology Jan Baedke notes that studies of epigenetic processes which are induced in the lab and those that are observed in nature are similar in that they share the same methodological framework. So we can use lab-induced epigenetic processes to ask evolutionary questions and get evolutionary answers in an epigenetic framework. There are two problems, though. One, that we don’t know whether experimental and natural epigenetic inducements will match up; and two we don’t know whether or not these epigenetic explanations that focus on proximate causes and not ultimate causes can address evolutionary explananda. Baedke (2018: 89) writes:
The first has been addressed by showing that studies of epigenetic processes that are experimentally induced in the lab (in molecular epigenetics) and those observed in natural populations in the field (in ecological or evolutionary epigenetics) are not that different after all. They share a similar methodological framework, one that allows them to pose heuristically fruitful research questions and to build reciprocal transparent models. The second issue becomes far less fundamental if one understands the predominant reading of Mayr’s classical proximate-ultimate distinction as offering a simplifying picture of what (and how) developmental explanations actually explain. Once the nature of developmental dependencies has been revealed, the appropriateness of developmentally oriented approaches, such as epigenetics, in evolutionary biology is secured.
Further arguments for epigenetics from an evolutionary approach can be found in Richardson’s (2017) Genes, Brains, and Human Potential (chapter 4 and 5) and Jablonka and Lamb’s (2005) Evolution in Four Dimensions. More than genes alone are passed on and inherited, and this throws a wrench into the MS.
Some may fault DST for not offering anything comparable to Darwinisim, as Dupre (2003: 37) notes:
Critics of DST complain that it fails to offer any positive programme that has achievements comparable to more orthodox neo-Darwinism, and so far this complaint is probably justified.
But this is irrelevant. For if we look at DST as just a part of the whole EES programme, then it is the EES that needs to—and does—“offer a positive programme that has achievements comparable to more orthodox neo-Darwinism” (Dupre, 2003: 37). And that is exactly what the EES does: it makes novel predictions; it explains what needs to be explained better than the MS; and the MS has shown to be incoherent (that is, there cannot be selection on only one level; there can only be selection on the organism). That the main tool of the MS (natural selection) has been shown by Fodor to be vacuous and non-mechanistic is yet another strike against it.
Since DST is a main part of the EES, and DST is “a wholeheartedly epigenetic approach to development, inheritance and evolution” (Griffiths, 2015) and the EES incorporates epigenetic theories, then the EES will live or die on whether or not its evolutionary epigenetic theories are confirmed. And with the recent slew of books and articles that attest to the fact that there is a huge component to evolutionary epigenetics (e.g., Baedke, 2018; Bonduriansky and Day, 2018; Meloni, 2019), it is most definitely worth seeing what we can find in regard to evolutionary epigenetics studies, since epigenetic changes induced in the lab and those that are observed in natural populations in nature are not that different. This can then confirm or deconfirm major hypotheses of the EES—of which there are many. It is time for Lamarck to make his return.
It is clear that the MS is lacking, as many authors have pointed out. To understand evolutionary history and why organisms have the traits they do, we need much more than the natural selection-dominated neo-Darwinian Modern Synthesis. We need a new synthesis (which has been formulated for the past 15-20 years) and only through this new synthesis can we understand the hows and whys. The MS was good when we didn’t know any better, but the reductionism it assumes is untenable; there cannot be any direct selection on any level (i.e., the gene) so it is a nonsensical programme. Genes are not directly selected, nor are traits that enhance fitness. Whole organisms and their developmental systems are selected and propagate into future generations.
The EES (and DST along with it) hold right to the causal parity thesis—“that genes/DNA play an important role in development, but so do other variables, so there is no reason to privilege genes/DNA above other developmental variables.” This causal parity between all tools of development is telling: what is selected is not just one level of the system, as genetic reductionists (neo-Darwinists) would like to believe; it occurs on the whole organism and what it interacts with (the environment); environments are inherited too. Once we purge the falsities that were forced upon us by the MS in regard to organisms and their relationship with the environment and the MS’s assumptions about evolution as a whole, we can then truly understand how and why organisms evolve the phenotypes they do; we cannot truly understand the evolution of organisms and their phenotypes with genetic reductionist thinking with sloppy logic. So who wins? The MS does not, since it has causation in biology wrong. This only leaves us with the EES as the superior theory, predictor, and explainer.
Book Review: “Lamarck’s Revenge”
3500 words
I recently bought Lamarck’s Revenge by paleobiologist Peter Ward (2018) because I went on a trip and needed something to read on the flight. I just finished the book the other day and I thought that I would give a review and also discuss Coyne’s review of the book since I know he is so uptight about epigenetic theories, like that of Denis Noble and Jablonka and Lamb. In Lamarck’s Revenge, Ward (2018) purports to show that Lamarck was right all along and that the advent of the burgeoning field is “Lamarck’s revenge” for those who—in the current day—make fun of his theories in intro biology classes. (When I took Bio 101, the professor made it a point to bring up Lamarck and giraffe necks as a “Look at this wrong theory”, nevermind the fact that Darwin was wrong too.) I will go chapter-by-chapter, give a brief synopsis of each, and then discuss Coyne’s review.
In the introduction, Ward discusses some of the problems with Darwinian thought and current biological understanding. The current neo-Darwinian Modern Synthesis states that what occurs in the lifetime of the organism cannot be passed down to further generations—that any ‘marks’ on the genome are then erased. However, recent research has shown that this is not the case. Numerous studies on plants and “simpler” organisms refute the notion, though for more “complex” organisms it has yet to be proved. However, that this discussion is even occurring is proof that we are heading in the right direction in regard to a new synthesis. In fact, Jablonka and Lamb (2005) showed in their book Evolution in Four Dimensions, that epigenetic mechanisms can and do produce rapid speciation—too quick for “normal” Darwinian evolution.
Ward (2018: 3-4) writes:
There are good times and bad times on earth, and it is proposed here that dichotomy has fueled a coupling of times when evolution has been mainly through Darwinian evolution and others when Lamarckian evolution has been dominant. Darwinian in good times, Lamarckian in bad, when bad can be defined as those times when our environments turn topsy-turvy, and do so quickly. When an asteroid hits the planet. When giant volcanic episodes create stagnant oceans. When a parent becomes a sexual predator. When our industrial output warms the world. When there are six billion humans and counting.
These examples are good—save the one about when a parent becomes a sexual predator (but if we accept the thesis that what we do and what happens to us can leave marks on our DNA that don’t change it but are passed on then it is OK)—and they all point to one thing: when the environment becomes ultra-chaotic. When such changes occur in the environment, that organism needs a physiology that is able to change on-demand to survive (see Richardson, 2017).
Ward (2018: 8) then describes Lamarck’s three-step process:
First, an animal experienced a radical change of the environment aroujnd it. Second, the initial response to the environmental change was some new kind of behavior by that of the animal (or whole species). Third, the behavioral change was followed by morphological change in subsequent generations.
Ward then discusses others before Darwin—Darwin’s grandfather Erasmus, for instance—who had theories of evolution before Darwin. In any case, we went from a world in which a God created all to a world where everything we see was created by natural processes.
Then in Chapter 2, Ward discusses Lamarck and Darwin and each of their theories in turn. (Note that Darwin did have Lamarckian views too.) Ward discusses the intellectual dual between Lamarck and Georges Cuvier, the father of the field of comparative anatomy—he studied mass extinctions. At Lamarck’s funeral, Cuvier spoke bad about Lamarck and buried his theories. (See Cuvier’s (1836) Elegy of Lamarck.) These types of arguments between academics have been going on for hundreds of years—and they will not stop any time soon.
In Chapter 3 Ward discusses Darwin’s ideas all the way to the Modern Synthesis, discussing how Darwin formulated his theory of natural selection, the purported “mechanism of evolution.” Ward discusses how Darwin at first rejected Lamarck’s ideas but then integrated them into future editions of On the Origin. We can think of this scenario: Imagine any environment and organisms in it. The environment rapidly shifts to where it is unrecognizable. The organisms in that environment then need to either change their behavior (and reproduce) or die. Now, if there were no way for organisms to change, say, their physiology (since physiology is dependent on what is occurring in the outside environment), then the species would die and there would be no evolution. However, the advent of evolved physiologies changed that. Morphologic and physiologic plasticity can and does help organisms survive in new environments—environments that are “new” to the parental organism—and this is a form of Lamarckism (“heritable epigenetics” as Ward calls it).
Chapter 4 discusses epigenetics and a newer synthesis. In the beginning of the chapter, Ward discusses a study he was a part of (Vandepas, et al, 2016). (Read Ward’s Nautilus article here.)
They studied two (so-called) different species of nautilus—one, nautilus pampilus, widespread across the Pacific and Indian Oceans and two, Nautilus stenomphalus which is only found at the Great Barrier Reef. Pompilus has a hole in the middle of its shell, whereas stenomphalus has a plug in the middle. Both of these (so-called) species have different kinds of anatomy—Pompilus has a hood covered with bumps of flesh whereas stenomphalus‘ hood is filled with projections of moss-like twig structures. So over a thirty-day period, they captured thirty nautiluses and snipped a piece of their tentacles and sequences the DNA found in it. They found that the DNA of these two morphologically different animals was the same. Thus, although the two are said to be different species based on their morphology, genetically they are the same species which leads Ward (2018: 52) to claim “that perhaps there are fewer, not more, species on Earth than science has defined.” Ward (2018: 53) cites a recent example—the fact that the Columbian and North American wooly mammoths “were genetically the same but the two had phenotypes determined by environment” (see Enk et al, 2011).
Now take Ward’s (2018: 58) definition of “heritable epigenetics”:
In heritable epigenetics, we pass on the same genome, but one marked (mark is the formal term for the place that a methyl molecule attaches to one nucleotide, a rung in the ladder of DNA) in such a way that the new organism soon has its own DNA swarmed by these new (and usually unwelcome) additions riding on the chromosomes. The genotype is not changed, but the genes carrying the new, sucker-like methyl molecules change the workings of the organism to something new, such as the production (or lack thereof) of chemicals necessary for our good health, or for how some part of the body is produced.
Chapter 5 discusses different environments in the context of evolutionary history. Environmental catastrophes that lead to the decimation of most life on the planet are the subject—something that Gould wrote about in his career (his concept of contingency in the evolutionary process). Now, going back to Lamarck’s dictum (first an environmental change, second a change in behavior, and third a change in phenotype), we can see that these kinds of processes were indeed imperative in the evolution of life on earth. Take the asteroid impact (K-Pg extinction; Cretaceous-Paleogene) that killed off the dinosaurs and threw tons of soot into the air, blocking out the sun making it effectively night (Schulte et al, 2010). All organisms that survived needed to eat. If the organism only ate in the day time, it would then need to eat at night or die. That right there is a radical environmental change (step 1) and then a change in behavior (step 2) which would eventually lead to step 3.
In Chapter 6, Ward discusses epigenetics and the origins of life. The main subject of the chapter is lateral gene transfer—the transmission of different DNA between genomes. Hundreds or thousands of new genes can be inserted into an organism and effectively change the morphology, it is a Lamarckian mechanism. Ward posits that there were many kinds of “genetic codes” and “metabolisms” throughout earth’s history, even organisms that were “alive” but were not capable of reproducing and so they were “one-offs.” Ward even describes Margulis’ (1967) theory of endosymbiosis as “a Lamarckian event“, which even Margulis accepts. Thus, the evolution of organisms is possible through lateral gene transfer and is another Lamarckian mechanism.
Chapter 7 discusses epigenetics and the Cambrian explosion. Ward cites a Creationist who claims that there has not been enough time since the 500 million year explosion to explain the diversity of body plans since then. Stephen Jay Gould wrote a whole book on this—Wonderful Life. It is true that Darwinian theory cannot explain the diversity of body plans, nor even the diversity of species and their traits (Fodor and Piatelli-Palmarini, 2010), but this does not mean that Creationism is true. If we are discussing the diversification of organismal life after mass extinctions, then Darwinian evolution cannot have possibly played a role in the survival of species—organisms with adaptive physiologies would have had a better chance of surviving in these new, chaotic environments.
It is posited here that four different epigenetic mechanisms presumably contributed to the great increase in both the kinds of species and the kinds of morphologies that distinguished them that together produced the Cambrian explosion as we currently know it: the first, now familiar, methylation; second, small RNA silencing; third, changes in the histones, the scaffolding that dictates the overall shape of a DNA molecule; and, finally, lateral gene transfer, which has recently been shown to work in animals, not just microbes. (Ward, 2018: 113)
Ginsburg and Jablonka (2010) state that “[associative] learning-based diversification was
accompanied by neurohormonal stress, which led to an ongoing destabilization and re-patterning of the epigenome, which, in turn, enabled further morphological, physiological, and behavioral diversification.” So associative learning, according to Ginsburg and Jablonka, was the driver of the Cambrian explosion. Ward (2018: 115) writes:
[The paper by Ginsburg and Jablonka] says that changes of behavior by both animal predators and animal prey began as an “arms race” in not just morphology but behavior. Learning how to hunt or flee; detecting food and mats and habitats at a distance from chemical senses of smell or vision, or from deciphering vibrations coming through water. Yet none of that would matter if the new behaviors and abilities were not passed on. As more animal body plans and the species they were composed of appeared, ecological communities changed radically and quickly. The epigenetic systems in snimals were, according to the authors, “destabilized,” andin reordering them it allowed new kinds of morphology, physiology, and again behavior, ans amid this was the ever-greater use of powerful hormone systems. Seeinf an approaching predator was not enough. The recognition of imminent danger would only save an animal’s life if its whole body was alerted and put on a “war footing” by the flooding of the creature with stress hormones. Poweful enactors of action. Over time, these systems were made heritable and, according to the authors, the novel evolution of fight or flight chemicals would have greatly enhanced survivability and success of early animals “enabled animals to exploit new niches, promoted new types of relations and arms races, and led to adaptive repsonses that became fixed through genetics.”
That, and vision. Brains, behavior, sense organs and hormones are tied to the nervous system to the digestive system. No single adaption led to animal success. It was the integration of these disparate systems into a whole that fostered survivability, and fostered the rapid evolution of new kinds of animals during the evolutionary fecund Cambrian explosion.
So, ever-changing environments are how physiological systems evolved (see Richardson, 2017: Chapters 4 and 5). Therefore, if the environment were static, then physiologies would not have evolved. Ever-changing environments were imperative to the evolution of life on earth. For if this were not the case, organisms with complex physiologies (note that a physiological system is literally a whole complex of cells) would never have evolved and we would not be here.
In chapter 8 Ward discusses epigenetic processes before and after mass extinctions. He states that, to mass extinction researchers, there are 3 ways in which mass extinction have occurred: (1) asteroid or comet impact; (2) greenhouse mass extinction events; and (3) glaciation extinction events. So these mass extinctions caused the emergence of body plans and new species—brought on by epigenetic mechanisms.
Chapter 9 discusses good and bad times in human history—and the epigenetic changes that may have occurred. Ward (2018: 149) discusses the Toba eruption and that “some small group of survivors underwent a behavioral change that became heritable, producing cultural change that is difficult to overstate.” Environmental change leads to behavioral change which eventually leads to change in morphology, as Lamarck said, and mass extinction events are the perfect way to show what Lamarck was saying.
In chapter 10 Ward discusses epigenetics and violence, the star of the chapter being MAOA. Take this example from Ward (2018: 167-168):
Causing violent death or escaping violent death or simply being subjected to intense violence causes significant flooding of the body with a whole pharmacological medicine chest of proteins, and in so doing changes the chemical state of virtually every cell. The produces epigenetic change(s) that can, depending on the individual, create a newly heritable state that is passed on to the offspring. The epigenetic change caused by the fight-or-flight response may cause progeny to be more susceptible to causing violence.
Ward then discsses MAOA (pg 168-170), though read my thoughts on the matter. (He discusses the role of epigenetics in the “turning on” of the gene. Child abuse has been shown to cause epigenetic changes in the brain (Zannas et al, 2015). (It’s notable that Ward—rightly—in this chapter dispenses with the nature vs. nurture argument.)
In Chapter 11, Ward discusses food and famine changing our DNA. He cites the most popular example, that of the studies done on survivors who bore children during or after the famine. (I have discussed this at length.) In September of 1944, the Dutch ordered a nation-wide railroad strike. The Germans then restricted food and medical access to the country causing the deaths of some 20,000 people and harming millions more. So those who were in the womb during the famine had higher rates of disorders such as obesity, anorexia, obesity, and cardiovascular incidences.
However, one study showed that if one’s father had little access to food during the slow growth period, then cardiovascular disease mortality was low. But diabetes mortality was high when the paternal grandfather was exposed to excess food. Further, when SES factors were controlled for, the difference in lifespan was 32 years, which was dependent on whether or not the grandfather was exposed to an overabundance of food or lack of abundance of food just before puberty.
Nutrition can alter the epigenome (Zhang and Kutateladze, 2018), since it can alter the epigenome and the epigenome is heritable, then these changes can be passed on to future generations too.
Ward then discusses the microbiome and epigenetics (read my article for a primer on the microbiome, what it does, and racial differences in it). The microbiome has been called “the second genome” (Grice and Segre, 2012), and so, any changes to the “second genome” can also be passed down to subsequent generations.
In Chapter 12, Ward discusses epigenetics and pandemics. Seeing people die from horrible diseases of course has horrible effects on people. Yes, there were evolutionary implications from these pandemics in that the gene pool was decreased—but what of the effects on the survivors? Methylation impacts behavior and behavior impacts methylation (Lerner and Overton, 2017), and so, differing behaviors after such atrocities can be tagged on the epigenome.
Ward then takes the discussion on pandemics and death and shifts to religion. Imagine seeing your children die, would you not want to believe that there was a better place for them after death to—somewhat—quell your sorrow over their loss? Of course, having an epiphany about something (anything, not just religon) can change how you view life. Ward also discusses a study where atheists had different brain regions activated even while no stimulation was presented. (I don’t like brain imaging studies, see William Uttal’s books and papers.) Ward also discusses the VMAT2 gene, which “controls” mood through the production of the VMAT protein, elevating hormones such as dopamine and serotonin (similar to taking numerous illegal drugs).
Then in Chapter 13 he discusses chemicals and toxins and how they relate to epigenetic processes. These kinds of chemicals and toxins are linked with changes in DNA methylation, miroRNAs, and histone modifications (Hou et al, 2012). (Also see Tiffon, 2018 for more on chemicals and how they affect the epigenome.)
Finally, in Chapter 14 Ward discusses the future of evolution in a world with CRISPR-CAS9. He discusses many ways in which the technology can be useful to us. He discusses one study in which Chinese scientists knocked out the myostatin gene in 65 dog embryos. Twenty-seven of the dogs were born and only two—a male and a female—had both copies of the myostatin gene disrupted. This is just like when researchers made “double-muscle” cattle. See my article ‘Double-Muscled’ Humans?
He then discusses the possibility of “supersoldiers” and if we can engineer humans to be emotionless killing machines. Imagine being able to engineer humans that had no sympathy, no empathy, that looked just like you and I. CRISPR is a tool that uses epigenetic processes and, thus, we can say that CRISPR is a man-made Lamarckian mechanism of genetic change (mimicking lateral gene transfer).
Now, let’s quickly discuss Coyne’s review before I give my thoughts on the book. He criticizes Ward’s article linked above (Coyne admits he did not read the book), stating that his claim that the two nautiluses discussed above being the same species with the same genome and epigenetic forces leading to differences in morphology (phenotype). Take Coyne’s critique of Vandepas, et al, 2016—that they only sequenced two mitochondrial genes. Combosch et al (2017; of which Ward was a coauthor) write (my emphasis):
Moreover, previous molecular phylogenetic studies indicate major problems with the conchiological species boundaries and concluded that Nautilus represents three geographically distinct clades with poorly circumscribed species (Bonacum et al, 2011; Ward et al, 2016). This is been reiterated in a more recent study (Vandepas et al, 2016), which concluded that N. pompilius is a morphologically variable species and most other species may not be valid. However, these studies were predominantly or exclusively based on mitochondrial DNA (mtDNA), an informative but often misleading marker for phylogenetic inference (e.g., Stöger & Schrödl 2013) which cannot reliably confirm and/or resolve the genetic composition of putative hybrid specimens (Wray et al, 1995).
Looks like Coyne did not look hard enough for more studies on the matter. In any case, it’s not just Ward that makes this argument—many other researchers do (see e.g., Tajika et al, 2018). So, if there is no genetic difference between these two (so-called) species, and they have morphological differences, then the possibility that seems likely is that the differences in morphology are environmentally-driven.
Lastly, Coyne was critical of Ward’s thoughts on the heritability of histone modification, DNA methylation, etc. It seems that Coyne has not read the work of philosopher Jan Baedke (see his Google Scholar page), specifically his book Above the Gene, Beyond Biology: Toward a Philosophy of Epigenetics along with the work of sociologist Maurizio Meloni (see his Google Scholar page), specifically his book Impressionable Biologies: From the Archaeology of Plasticity to the Sociology of Epigenetics. If he did, Coyne would then see that his rebuttal to Ward makes no sense as Baedke discusses epigenetics from an evolutionary perspective and Meloni discusses epigenetics through a social, human perspective and what can—and does—occur in regard to epigenetic processes in humans.
Coyne did discuss Noble’s views on epigenetics and evolution—and Noble responded in one of his talks. However, it seems like Coyne is not aware of the work of Baedke and Meloni—I wonder what he’d say about their work? Anything that attacks the neo-Darwinian Modern Synthesis gets under Coyne’s skin—almost as if it is a religion for him.
Did I like the book? I thought it was good. Out of 5 stars, I give it 3. He got some things wrong, For instance, I asked Shea Robinson, author of Epigenetics and Public Policy: The Tangled Web of Science and Politics about the beginning of the book and he directed me to two articles on his website: Lamarck’s Actual Lamarckism (or How Contemporary Epigenetics is not Lamarckian) and The Unfortunate Legacy of Jean-Baptiste Lamarck. The beginning of the book is rocky, the middle is good (discussing the Cambrian explosion) and the end is alright. The strength of the book is how Ward discusses the processes that epigenetics occurs by and how epigenetic processes can occur—and help drive—evolutionary change, just as Jablonka and Lamb (1995, 2005) argue, along with Baedke (2018). The book is a great read, if only for the history of epigenetics (which Robinson (2018) goes into more depth, as does Baedke (2018) and Meloni (2019)).
Lamarck’s Revenge is a welcome addition to the slew of books and articles that go against the Modern Synthesis and should be required reading for those interested in the history of biology and evolution.
Race, Medicine, and Epigenetics: How the Social Becomes Biological
2050 words
Race and medicine is a tendentious topic. On one hand, you have people like sociologist Dorothy Roberts (2012) who argues against the use of race in a medical context, whereas philosopher of race Michael Hardimon thinks that we should not be exclusionists about race when it comes to medicine. If there are biological races, and there are salient genetic differences between them, then why should we disregard this when it comes to a medically relevant context? Surely Roberts would agree that we use her socio-political concept of race when it comes to medicine, but not treat them like biological races. Roberts is an anti-realist about biological races, whereas Hardimon is not—he recognizes that there is a minimalist and social aspect to race which are separate concepts.
In his book Rethinking Race: The Case for Deflationary Realism, Hardimon (2017, Chapter 8) discusses race and medicine after discussing and defending his different race concepts. If race is real—whether socially, biologically, or both—then why should we ignore it when it comes to medical contexts? It seems to me that many people would be hurt by such a denial of reality, and that’s what most people want to prevent, and which is the main reason why they deny that races exist, so it seems counterintuitive to me.
Hardimon (2017: 161-162; emphasis his) writes:
If, as seems to be the case, the study of medically relevant genetic variants among races is a legitamate project, then exclusionism about biological race in medical research—the view that there is no place for a biological concept of race in medical research—is false. There is a place for a biological concept of race in the study of medically relevant genetic variants among races. Inclusionism about biological race in medical research is true.
So, we should not be exclusionists (like Roberts), we should be inclusionists (like Hardimon). Sure, some critics would argue that we should be looking at the individual and not their racial or ethnic group. But consider this: Imagine that an individual has something wrong and standard tests do not find out what it is. The doctor then decides that the patient has X disease. They then treat X disease, and then find out that they have Y disease that a certain ethnic group is more likely to have. In this case, accepting the reality of biological races and its usefulness in medical research would have caught this disease earlier and the patient would have gotten the help they needed much, much sooner.
Black women are more likely to die from breast cancer, for example, and racism seems like it can explain a lot of it. They have less access to screening, treatment, care, they receive delays in diagnoses, along with lower-quality treatment than white women. But “implicit racial bias and institutional racism probably play an important role in the explanation of this difficult treatment” (Hardimon, 2017: 166). Furthermore, black women are more than twice as likely to acquire a type of breast cancer called “triple negative” breast cancer (Stark et al, 2010; Howlader et al, 2014; Kohler et al, 2015; DeSantis et al, 2019). Of course, this could be a relevant race-related genetic difference in disease.
We should, of course, use the concepts of socialrace when discussing the medical effects of racism (i.e., psychosocial stress) and the minimalist/populationist race concepts when discussing the medically relevant race-related genetic diseases. Being eliminativist about race doesn’t make sense—since if we deny that race exists at all and do not use the term at all anymore, there would be higher mortality for these “populations.” Thus, we should use both of Hardimon’s terms in regard to medicine and racial differences in health outcomes as both concepts can and will show us how and why diseases are more likely to appear in certain racial groups; we should not be eliminativists/exclusionists about race, we should be inclusionists.
Hardimon discusses how racism can manifest itself as health differences, and how this can have epigenetic effects. He writes (pg 155-156):
As philosopher Shannon Sullivan explains, another way in which racism may be shown to influence health is by causing epigenetic changes in the genotype. It is known that changes in gene expression can have durable and even transgenerational effects on health, passing from parents to their children and their children’s children. This suggests that the biological dimensions of racism can replicate themselves across more than one generation through epigenetic mechanisms. Epigenetics, the scientific study of such changes, explains how the process of transgenerational biological replication of ill health can occur without changes in the underlying DNA sequence.
If such changes to the DNA sequences can be transmitted to the next generation in the developmental system, then that means that the social can—and does—has an effect on our biology and that it can be passed down through subsequent generations. It is simple to explain why this makes sense: for if the developing organism was not plastic, and genes could not change based on what occurs in the environment for the fetus or the organism itself, then how could organisms survive when the environment changes if the “genetic code” of the genome were fixed and not malleable? For example, Jasienska (2009) argues that:
… the low birth weight of contemporary African Americans not only results from the difference in present exposure to lifestyle factors known to affect fetal development but also from conditions experienced during the period of slavery. Slaves had poor nutritional status during all stages of life because of the inadequate dietary intake accompanied by high energetic costs of physical work and infectious diseases. The concept of ‘‘fetal programming’’ suggests that physiology and metabolism including growth and fat accumulation of the developing fetus, and, thus its birth weight, depend on intergenerational signal of environmental quality passed through generations of matrilinear ancestors.
If the environmental quality—i.e., current environmental quality—is “known” by the developing fetus through cues from the mother’s nutrition, stress etc, then a smaller body size may be adaptive in that certain environment and the organism may survive with fewer resources due to smaller body size. In any case, I will discuss this in the future but it was just an example of a possible epigenetic modification on current slaves. I, personally, have noticed that a lot of blacks are really skinny and have really low body fat—who knows, maybe this could be part of the reason why?
This is something that sociologist Maurizio Meloni (2018) calls “the postgenomic body”—the fact that biology is malleable through what occurs in our social lives. So not only is the human brain plastic, but so is the epigenome and microbiome, which is affected by diet and lifestyle—along with what we do and what occurs to us in our social lives. So our social lives, in effect, can become embodied in our epigenome and passed down to subsequent generations. Similar points are also argued by Ulijaszek, Mann, and Elton (2012). (Also see my article Nutrition, Development, Epigenetics, and Physical Plasticity.)So in effect, environments are inherited too, and so, therefore, the environments that we find ourselves in are, in effect, passed down through the generations. Meloni (2018) writes:
On the other hand, by re-embedding the individual within a wider lineage of ancestral experiences and reconfiguring it as a holobiontic assemblage, it may literally dissolve the subject of emancipation. Moreover, the power of biological heredity may be so expanded (as it includes potentially any single ancestral experience) to become stronger than in any previous genetic view. Finally, the several iterations of plasticity that emerge from this genealogy appear so deeply racialized and gendered that it is difficult to quickly turn them into an inherently emancipatory concept. Even as a concept, plasticity has an inertial weight and viscosity that is the task of the genealogist to excavate and bring into view.
Thus, current biological states can be “tagged” and therefore be epigenetically transmitted to future generations. Think about it in this way: if epigenetic tags can be transmitted to the next generation then it would be presumed that that environment—or a similar one—would be what newer generations would be born in. Thus, the plasticity of the organism would help it in life, especially the immediate plasticity of the organism in the womb. Likewise, Kuzawa and Sweet (2008) argue:
that environmentally responsive phenotypic plasticity, in combination with the better-studied acute and chronic effects of social-environmental exposures, provides a more parsimonious explanation than genetics for the persistence of CVD disparities between members of socially imposed racial categories.
Of course, if we look at race as both a biological and social category (i.e., Spencer, 2014), then this is not surprising that differences in disease acquisition can persist “between members of socially imposed racial categories.” Phenotypic plasticity is the big thing here, as noted by many authors who write about epigenetics. If the organism is plastic (if it can be malleable and change depending on external environmental cues), then disease states can—theoretically—be epigenetically passed to future generations. This is just like Jasienska’s (2009, 2013, Chapter 5) argument that the organism—in this case, the fetus—can respond to the environmental quality that it is developing in and, therefore, differences in anatomy and physiology can and do occur based on the plasticity of the organism.
Lastly, Jan Badke, author of Above the Gene, Beyond Biology: Toward a Philosophy of Epigenetics (Baedke, 2018), argues that, since the gene-centered view of biology has been upended (i.e., Jablonka and Lamb, 2005; Noble, 2006, 2011, 2012, 2017) for a postgenomic view (Richardson and Stevens, 2015). Genes are not closed off from the environment; all organisms, including humans, are open systems and so, there are relationships between the environment, developmental system, and the genome which affect the developing organism. Baedke and Delgado (2019) argue that the “colonial shadow … biologicizes as well as racializes social-cultural differences among human groups.” Since every race faces specific life challenges in its environment, therefore, each race shows a “unique social status that is closely linked to its biological status.” Thus, differing environments, such as access to different foods (i.e., the effects of obesifying foods) and discrimination can and are passed down epigenetically. Baedke and Delgado (2019: 9) argue that:
… both racial frameworks nutrition plays a crucial role. It is a key pathway over which sociocultural and environmental difference are embodied as racial difference. Thus, belonging to a particular race means having a particular biosocial status, since races include two poles – a social status (e.g., class, socio-economic status) and a biological status (disease susceptibility) – which are closely interlinked. Against this background, human populations in Mexico become an exemplar of types of bodies that are not only relocated to a destabilizing modernized world in which they suffer from socio-economic deprivation. What is more, they become paradigmatic primitive bodies that are unbalanced, biologically deprived, and sick. In short, in these recent epigenetic studies poor places and lifestyles determine poor bodies, and vice versa.
In sum, accepting the reality of race—both in a minimalist/populationist biological manner and social manner—can and will help us better understand disease acquisition and differing levels of certain diseases between races. Recognizing the minimalist/populationist concepts of race will allow us to discover genetic differences between races that contribute to variation in different diseases—since genes do not alone outright cause diseases (Kampourakis, 2017: 19). Being eliminativist/exclusionist about race does not make sense, and it would cause much more harm than good when it comes to racial disease acquisition and mortality rates.
Furthermore, acknowledging the fact that the social dimensions of race can help us understand how racism manifests itself in biology (for a good intro to this see Sullivan’s (2015) book The Physiology of Racist and Sexist Oppression, for even if the “oppression” is imagined, it can still have very real biological effects that could be passed onto the next generation—and it could particularly affect a developing fetus, too). It seems that there is a good argument that the effects of slavery could have been passed down through the generations manifesting itself in smaller bodies; these effects also could have possibly manifested itself in regard to obesity in Latin America post-colonialism. Gravlee (2009) and Kaplan (2010) also argue that the social, too, manifests itself in biology.
(For further information on how the social can and does become biological see Meloni’s (2019) book Impressionable Biologies: From the Archaeology of Plasticity to the Sociology of Epigenetics, along with Meloni (2014)‘s paper How biology became social, and what it means for social theory. Reading Baedke’s and Meloni’s arguments on plasticity and epigenetics should be required before discussing these concepts.)
Gene-Selectionism vs. Developmental Systems Theory
2300 words
Two dominant theories exist in regard to development, the “gene’s eye view—gene selectionism (GS)—and the developmental view—developmental systems theory (DST). GS proposes that there are two fundamental processes in regard to evolution: replication and interaction. Replicators (the term was coined by Dawkins) are anything that is copied into the next generation whereas interactors (vehicles) are things that only exist to ensure the replicators’ survival. Thus, Dawkins (1976) proposes a distinction between the “vehicle” (organism) and its “riders/replicators” (the genes).
Gene selectionism
Gene selectionists propose a simple hypothesis: evolution through the differential survival of genes, its main premise being that the “gene” is “the ultimate, fundamental unit of natural selection.” Dusek (1999: 156) writes that “Gene selection claims that genes, not organisms, groups of organisms or species, are selected. The gene is considered to be the unit of selection.” The view of gene selectionists is best—and most popularly put—by Richard Dawkins’ seminal book The Selfish Gene (1976), in which he posits that genes “compete” with each other, and that our “selfish actions” are the result of our genes attempting to replicate to the next generation, relegating our bodies to disposable “vehicles” that only house the “replicators” (or “drivers).
Though, just because one is a gene selectionist does not necessarily mean that they are a genetic determinist (both views will be argued below). Gene selectionists are comitted to the view that genes make a distinctive contribution toward building interactors. Dawkins (1982) claims that genetic determinism is not a problem in regard to gene selectionism. Replicators (genes) have a special status to gene selectionists. Gene selectionists argue that adaptive evolution only occurs through cumulative selection, while only the replicators persist through the generations. Gene selectionists do not see organisms as replicators since genes—and not organisms—are what is replicated according to the view.
The gene selectionist view (Dawkins’ 1976 view) can also be said to apply what Okasha (2018) terms “agential thinking”. “Agential thinking” is “treating an evolved organism as if it were an agent pursuing a goal, such as survival or reproduction, and treating its phenotypic traits, including its behaviour, as strategies for achieving that goal, or furthering its biological interests” (Okasha, 2018: 12). Dawkins—and other gene selectionists—treat genes as if they have agency, speaking of “intra-genomic conflict”, as if genes are competing with each other (sure, it’s “just a metaphor”, see below).
Okasha (2018: 71) writes:
To see how this distinction relates to agential thinking, note that every gene is necessarily playing a zero-sum game against other alleles at the same locus: it can only spread in the population if they decline. Therefore every gene, including outlaws, can be thought of as ‘trying’ to outcompete alleles, or having this as its ultimate goal.
Selfish genes also have intermediate goals, which are to maximize fitness, which is done through expression in the organismic phenotype.
Thus, according to Okasha (2018: 73), “… selfish genetic elements have phenotypic effects which can be regarded as adaptations, but only if we apply the notions of agent, benefit, and goal to genes themselves”, though “… only in an evolutionary context [does] it [make] sense to treat genes as agent-like and credit them with goals and interests.” It does not “make sense to treat genes as even “agent-like and credit them with goals and interests since they can only be attributed to humans.
Other genes have as their intermediate goal to enhance the fitness of their host organism’s relatives, by causing altruistic behaviour [genes can’t cause altruistic behavior; it is an action]. However, a small handful of genes have a different intermediate goal, namely to increase their own transmission in their host organism’s gametes, for example, by biasing segregation in their favour, or distorting the sex-ratio, or transposing to new sites in the genome. These are outlaws, or selfish genetic elements.If oulaws are absent or are effectively suppressed, then the genes within a single organism have a common (intermediate) goal, so will cooperate: each gene can onluy benefit by itself by benefiting the whole organism. Agential thinking then can be applied to the organism itself. The organism’s goal—maximizing its fitness—then equates to the intermediate goal of each of the genes within it. (Okasha, 2018: 72)
Attributing agential thinking to anything other than humans is erroneous, since genes are not “selfish.”
The selfish gene is one of the main theories that define the neo-Darwinian paradigm and it is flat out wrong. Genes are not ultimate causes, as the crafters of the neo-Darwinian Modern Synthesis (MS) propose, genes are resources in a dynamic system and can thusly only be seen as causes in a passive, not active, sense (Noble, 2011).
Developmental systems
The alternative to the gene-centric view of evolution is that of developmental systems theory (DST), first proposed by Oyama (1985).
The argument for DST is simple:
(1) Organisms obviously inherit more than DNA from their parents. Since organisms can behave in ways that alter the environment, environments are also passed onto offspring. Thus, it can be said that genes are not the only things inherited, but a whole developmental matrix is.
(2) Genes, according to the orthodox view of the MS, interact with many other factors for development to occur, and so genes are not the only thing that help ‘build’ the organism. Genes can still play some “privileged” role in development, in that they “control”, “direct” or “organize” everything else, but this is up to gene-selectionists to prove. (See Noble, 2012.)
(3) The common claim that genes contain “information” (that is, context-independent information) is untenable, since every reconstruction of genes contain development about information applies directly to all other developmental outcomes. Genes cannot be singled out as privileged causes in development.
(4) Other attempts—such as genes are copied more “directly—are mistaken, since they draw a distinction between development and other factors but fail.
(5) Genes, then, cannot be privileged in development, and are no different than any other developmental factor. Genes, in fact, are just passive templates for protein construction, waiting to be used by the system in a context-dependent fashion (see Moore, 2002; Schneider, 2007). The entire developmental system reconstructs itself “through numerous independent causal pathways” (Sterelny and Griffiths, 1999: 109).
DNA is not the only thing inherited, and the so-called “famed immortality of DNA is actually a property of cells [since] [o]nly cells have the machinery to correct frequent faults that occur in DNA replication.” The thing about replication, though, is that “DNA and the cell must replicate together” (Noble, 2017: 238). A whole slew of developmental tools are inherited and that is what constructs the organism; organisms are, quite obviously, constructed not by genes alone.
Developmental systems, as described by Oyama (1985: 49) do not “have a final form, encoded before its starting point and realized at maturity. It has, if one focuses finely enough, as many forms as time has segments.” Oyama (1985: 61) further writes that “The function of the gene or any other influence can be understood only in relation to the system in which they are involved. The biological relevance or any influence, and therefore the very “information” it conveys, is jointly determined, frequently in a statistically interactive, not additive, manner, by that influence and the system state it influences.”
DNA is, of course, important. For without it, there would be nothing for the cell to read (recall how the genome is an organ of the cell) and so no development would occur. DNA is only “information” about an organism only in the process of cellular functioning.
The simple fact of the matter is this: the development of organs and tissues are not directly “controlled” by genes, but by the exchange signals of the cells. “Details notwithstanding, what is important to note is that whatever kinds of signals it sends out depends on the kind of signals it receives from its immediate environment. Therefore, neighboring cells are interdependent, and its local interactions among cells that drive the developmental processes” (Kampourakis, 2017: 173).
The fact of the matter is that whether or not a trait is realized depends on the developmental processes (and the physiologic system itself) and the environment. Kampourakis, just like Noble (2006, 2012, 2017) pushes a holistic view of development and the system. Kampourakis (2017: 184) writes:
What genetics research consistently shows is that biological phenomena should be approached holistically. at various levels. For example, as genes are expressed and produce proteins, and some of these proteins regulate or affect gene expression, there is absolutely no reason to privilege genes over proteins. This is why it is important to consider developmental processes in order to undertand how characters and disease arise. Genes cannot be considered alone but only in the broader context (cellular, organismal, environmental) in which they exist. And both characters and disease in fact develop; they are not just produced. Therefore, reductionism, the idea that genes provide the ultimate explanation for characters and disease, is also wrong. In order to understand such phenomena, we need to consider influence at various levels of organization, both bottom-up and top-down. This is why current research has adopted a systems biology approach (see Noble, 2006; Voit, 2016 for accessible introductions).
All this shows that developmental processes and interactions play a major role in shaping characters. Organisms can respond to changing environments through changes in their development and eventually their phenotypes. Most interestingly, plastic responses of this kind can become stable and inherited by their offspring. Therefore, genes do not predetermine phenotypes; genes are implicated in the development of phenotypes only through their products, which depends on what else is going on within and outside cells (Jablonka, 2013). It is therefore necessary to replacr the common representation of gene function presented in Figure 9.6a, which we usually find in the public sphere, with others that consider development, such as the one in figure 9.6b. Genes do not determine characters, but they are implicated in their development. Genes are resources that provide cells with a generative plan about the development of the organism, and have a major role in this process through their products. This plan is the resouce for the production of robust developmental outcomes that are at the same time plastic enough to accomodate changes stemming from environmental signals.
Figure 9.6 (a) The common representation of gene function: a single gene determines a single phenotype. It should be clear by what has been present in the book so far that is not accurate. (b) A more accurate representation of gene function that takes development and environment into account. In this case, a phenotype is propduced in a particular environment by developmental processes in which genes are implicated. In a different environment the same genes might contribute tothe development of a different phenotype. Note the “black box” of development.
[Kampourakis also writes on page 188, note 3]
In the original analogy, Wolpert (2011, p. 11) actually uses the term “program.” However, I consider the term “plan” as more accurate and thus more appropriate. In my view, the term “program” impies instructions and their implimentation, whereas the term “plan” is about instructions only. The notion of a genetic program can be very misleading because it implies that, if it were technically feasible, it would be possible to compute an organism by reading the DNA sequence alone (see Lewontin, 2000, pp. 17-18).
Kampourakis is obviously speaking of a “plan” in a context-dependent manner since that is the only way that genes/DNA contain “information” (Moore, 2002; Schneider, 2007). The whole point is that genes, to use Noble’s terminology, are “slaves” to the system, since they are used by and for the (physiological) system. Developmental systems theory is a “wholeheartedly epigenetic approach to development, inheritance and evolution” (Hochman and Griffiths, 2015).
This point is driven home by Richardson (2017:111):
And how did genes eventually become established? Probably not at all as the original recipes, designers, and controllers of life. Instead they arose as templates for molecular components used repeatedly in the life of the cell and the organism: a kind of facility for just-in-time production of parts needed on a recurring basis. Over time, of course, the role of these parts themselves evolved to become key players in the metabolism of the call—but as part of a team, not the boss.
[…]
It is not surprising, then, that we find that variation in form and function has, for most traits, only a tenuous relationship with variation in genes.
[And also writes on page 133]:
There is no direct command line between environments and genes or between genes and phenotypes. Predictions and decisions about form and variation are made through a highly evolved dynamical system. That is why ostensibly the same environment, such as hormonal signal, can initiate a variaety of responses like growth, cell division, differentiation, and migration, depending on deeper context. This reflects more than fixes responses from fixed information in genes, something fatally overlooked in the nature-nurture debate
(Also read Richardson’s article So what is a gene?)
Conclusion
The gene-selectionist point-of-view entails too many (false) assumptions. The DST point of view, on the other hand, does not fall prey to the pitfalls of the gene-selectionist POV; Developmental systems theorists look at the gene, not as the ultimate causes of development—and, along with that, only changes in gene frequency driving evolutionary change—but only as products to be used by and for the system. Genes can only be looked at in terms of development, and in no other way (Kamporuakis, 2017; Noble, 2017). Thus, the gene-selectionists are wrong; the main tenet of the neo-Darwinian Modern Synthesis, gene-selectionism—the selfish gene—has been refuted (Jablonka and Lamb, 2005; Noble, 2006, 2011). The main tenets of the neo-Darwinian Modern Synthesis have been refuted, and so it is now time to replace the Modern Synthesis with a new view of evolution: one that includes the role of genes and development and the role of epigenetics on the developmental system. The gene-selectionist view champions an untenable view of the gene: that the gene is priviliged above any other developmental variables, but Noble and Kampourakis show that this is not the case, since DNA is inherited with the cell; the cell is what is “immortal” to use the language of Dawkins—not DNA itself.
A priori, there is no privileged level of causation, and this includes the gene, which so many place at the top of the hierarchy (Noble, 2012).
Nutrition, Development, Epigenetics, and Physical Plasticity
1650 words
Humans are extremely “plastic”. “Plastic” meaning that our development can be shaped by what goes on (or does not go in) in our developmental environment along with the environment outside of the womb. Many factors drive development, and if one factor changes then part of the developmental course for the organism changes as well. Thus, environment can and does drive development, with the addition (or subtraction) of different factors. In this article, I will discuss some of the factors that drive development and physical plasticity and what can change them.
Subsistence provides food while food provides nutrition. Nutrients, then, supply our bodies with energy and promote tissue growth—among other things. However, nutrient requirements vary across and between species, while all mammals need a mixture of macronutrients (carbs, fat, protein, water, and fiber) and micronutrients (vitamins and minerals). Biological variability in nutrient requirements and “the eventual degree of metabolic function that an individual can achieve for a particular intake level is determined to a greater or lesser extent by genetic variants in enzymes controlling the absorption, uptake, distribution, retention or utilization of the nutrient” (Molloy, 2004: 156). Thus, individuals who consume the same amount of micro and macronutrients—who also have different polymorphisms in genes coding for the metabolism of any nutrient (through hormones and enzymes)—can, and do, have differing physiological responses to same vitamin intake. Thus, differences in genetic polymorphisms between individuals can—and do—lead to different disease.
Next we have phenotypic plasticity. Phenotypic plasticity, simply put, is the ability for a genome to express a different phenotype in variable environments. For instance, people born in hotter environments—no matter their race or ethnicity—develop larger pores in order to sweat more, since sweating is needed for cooling the body (Lieberman, 2015). Phenotypic plasticity can be a problem, though, in environments with numerous environmental stressors that will stress the mother and, in turn, affect the baby’s development in the womb as well affecting post-birth events. An example of this is when food availability is low and exposure to infection is high (in-utero and post-birth), and when these stressors are removed, the organism in question shows “catch-up growth”, implying that these stressors impeded the development of the organism in question.
Maternal nutritional imbalance has been found—both in animal studies and epidemiological studies—and metabolic disturbances, during critical windows of development for the organism, have both a persistent effect on the health of the organism and can be transmitted epigenetically to future generations (Gallou-Kabani and Junien, 2005). Gallou-Kabani and Junien (2005) write:
Epigenetic chromatin marks may be propagated mitotically and, in some cases, meiotically, resulting in the stable inheritance of regulatory states. Transient nutritional stimuli occurring at critical ontogenic stages may have lasting influences on the expression of various genes by interacting with epigenetic mechanisms and altering chromatin conformation and transcription factor accessibility (11).
Thus, metabolic syndrome can show transgenerational effects by way of incomplete erasure of the epigenetic factors carried by grandparents and parents. (See also Treretola et al, 2005.) Epigenetic regulation was extremely important during our evolution and especially during the development of the human organism, and is how and why we are so phenotypically plastic.
Epigenetic regulation during fetal reprogramming of the individual in preparation for the environment they expect to enter is likely to be a response to seasonal energy imbalance; changes that favour the metabolic efficiency are likely to be adaptive in such circumstances. Removal of seasonal energy stress, as has taken place in contemporary industrialized societies, may turn efficiency toward pathology. Humans thus have evolved an animal model that can respond genetically (through natural selection), phenotypically (through developmental plasticity) and epigenetically (by a balance of both). (Ulijaszek, Mann, and Elton, 2013: 19)
This seems to be a fundamental response to the human organism in-utero, responding to the lack of food in its environment and growing accordingly (low birth weight, susceptibilities to differing disease), which are still a problem for much of the developed world. Though this can be maladaptive in the developed, industrialized world, since poor early-life environments can lead to epigenetic changes which then spell out bad consequences for the low-birth-weight baby who was exposed to a slew of negative nutritional factors during conception (and post-birth).
It has already been established that nutrition can alter the genome and epigenome (Niculescu and Lupu, 2011; Niculescu, 2012; Anderson, Sant, and Dolinoy, 2012). So if differing nutritional effects can alter the genome and epigenome and these effects are transgenerationally inherited by future generations, then famines change the expression of the genome and epigenome which can then inherited by future generations if the epigenetic factors carried by the grandparents and parents are not erased (and there is mounting evidence for this claim, see Yang, Liu, and Sun, 2017).
There is evidence of phenotypic plasticity regarding the lack of nutrition when it comes to humans, in-utero, and the evidence comes from the Dutch Family Studies (see Lumey et al, 2007 for an overview of the project). Individuals who were prenatally exposed to the Dutch winter famine of 1944-45, six decades later, had less DNA methylation of the IGF2 (insulin-like growth factor 2) gene than same-sex siblings who were not exposed to the winter famine (Heijmns et al, 2008). The IGF2 gene plays an essential role of the development of the fetus before birth. The gene is highly active during fetal development, but much less so after birth. (It should be noted that the loss of imprinting on the IGF2 gene can promote prostate cancer; Fenner, 2017 and loss of imprinting on IGF2 can also promote other types of cancer as well; Livingstone, 2013).
Stein et al (2009) concluded that “famine exposure prior to conception is associated with poorer self-reported mental health and a higher level of depressive symptoms.” Tobi et al (2009) write that their data “support the hypothesis that associations between early developmental conditions and health outcomes later in life may be mediated by changes in the epigenetic information layer.” Tobi et al (2014) also show that the “Epigenetic modulation of pathways by prenatal malnutrition may promote an adverse metabolic phenotype in later life.” The prenatal—and neonatal—periods of development are of utmost importance in order for the organism to develop normally, any deviation outside of these measures can—and does—affect the genome and epigenome (Hajj et al, 2014).
Another strong example that these responses are adaptive to the organism in question is the fact that people who were exposed to nutritional imbalances in the womb showed a higher chance of becoming obese later in life (Roseboom, de Rooji, and Painter, 2006). Their study has implications for babies born in developing countries (since famines mirror, in a way, developing countries). Roseboom, de Rooji, and Painter (2006: 489) write:
This may imply that adaptations that enable the fetus to continue to grow may nevertheless have adverse consequences for health in later life.
Roseboom, de Rooji, and Painter (2006: 490) also write:
The nutritional experience of babies who were exposed to famine in early gestation may resemble that of babies in developing countries whose mothers are undernourished in early pregnancy and receive supplementation later on, but also of babies in developed countries whose mothers suffer from severe morning sickness.
So on-going studies, such as the Dutch Famine Study, have the chance to elucidate the mechanisms of low birth weight, and it can also show us how and why those exposed to adverse conditions in the womb show so many negative symptoms which are not present in kin who were not exposed to such malnutrition in the womb. These findings also suggest that nutrition before—and after—pregnancy can play a role in disease acquisition later in life. The fact that those exposed to famines have a higher chance of becoming obese later in life (Abeleen et al, 2012; Meng et al, 2017) shows that this adaptive response of the organism in the womb was very important in our evolution; the babe exposed to low maternal nutrition in the womb can, after birth, consume enough energy to become overweight, which would have been an adaptive evolutionary response to low maternal caloric energy.
Babies who are exposed to maternal under-nutrition in the womb—when exposed to an environment with ample foodstuffs—are at heightened risk of becoming type II diabetics and acquiring metabolic syndromes (Robinson, Buchholz, and Mazurak, 2007). This seems to be an adaptive, plastic response of the organism: since nutrients/energy were in low quantity in the womb, low nutrients/energy in the womb changed the epigenome of the organism, and so when (if) the organism is exposed to an environment with ample amounts of food energy, they will then have a higher susceptibility to metabolic syndromes and weight gains, due to their uterine environment. (Diet also has an effect on brain plasticity in both animals and humans, in the womb and out of it; see Murphy, Dias, and Thuret, 2014.)
In sum, phenotypic plasticity, which is driven in part by epigenetics, was extremely important in our evolution. This epigenetic regulation that occurs in the womb prepared the individual in question to be able to respond to the energy imbalance of the environment the organism was born in. The plasticity of humans, and animals, in regard to what occurs (or does not occur) in the environment, is how we were able to survive in new environments (not ancestral to our species). Epigenetic changes that occur in the grandparental and parental generations, when not completely erased during the meiotic division of cells, can affect future generations of progeny in a negative way.
The implications of the data are clear: under-nutrition (and malnutrition) affect the genome and epigenome in ways that are inherited through the generations, which is due to the physical plasticity of the human in-utero as well as post-birth when the baby developing. These epigenetic changes then lead to the one who experienced the adverse uterine environment to have a higher chance of becoming obese later in life, which suggests that this is an adaptive response to low amounts of nutrients/caloric energy in the uterine environment.